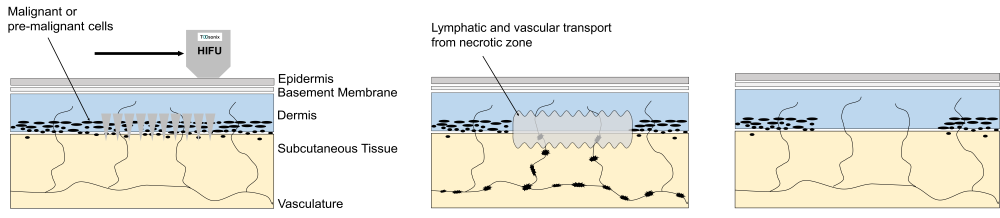
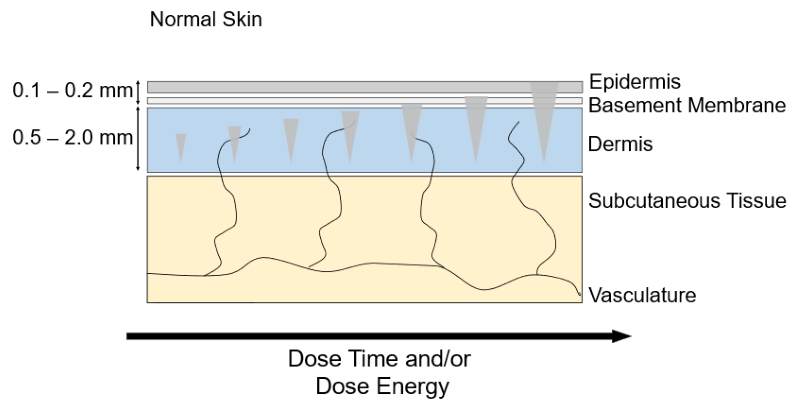
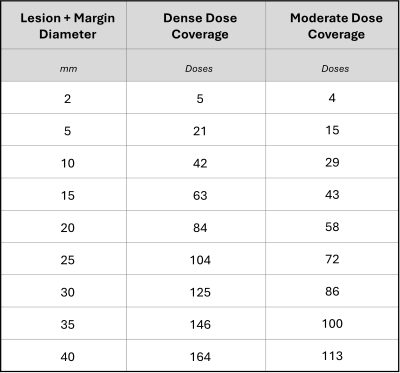
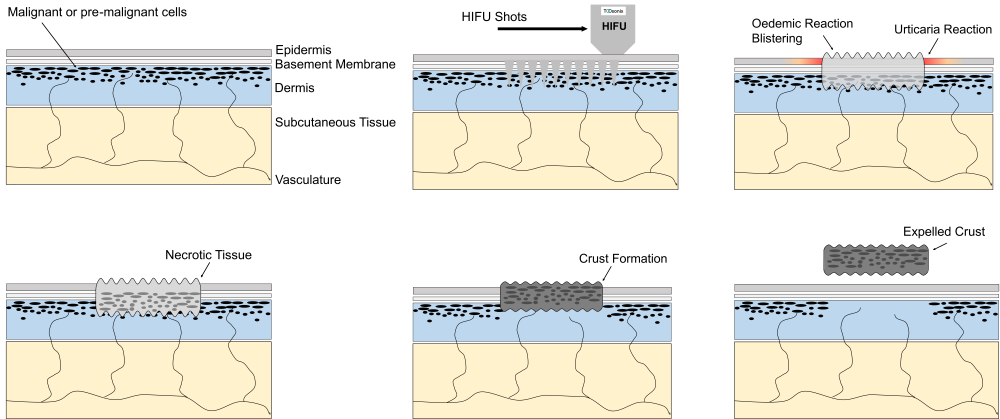
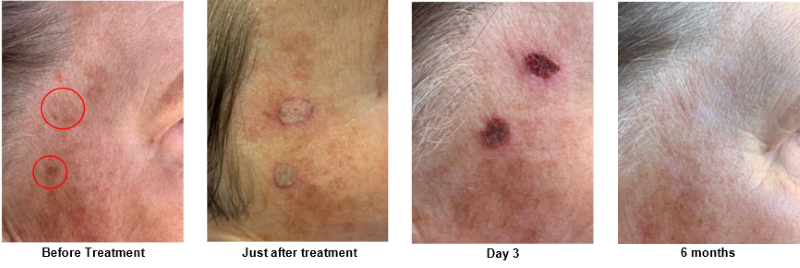
HIFU Energy
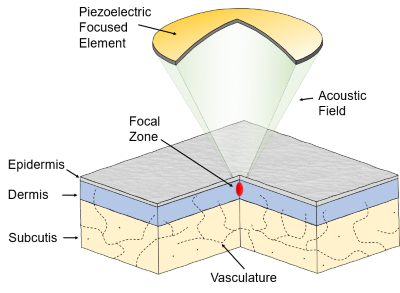
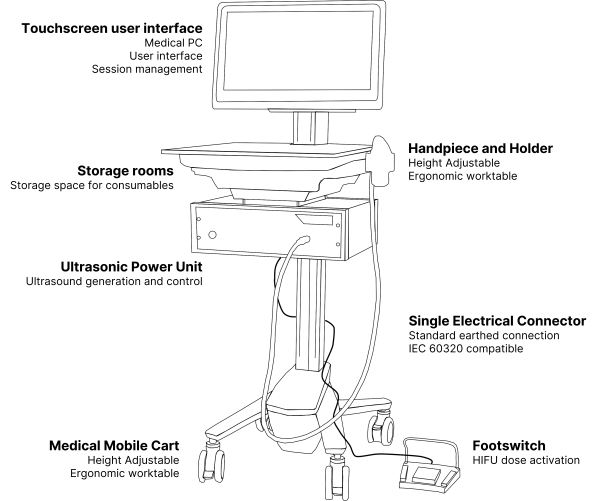
The TOOsonix system converts electrical energy from the power grid to an acoustic ultrasound signal emitted from the ultrasound transducer in the handpiece.
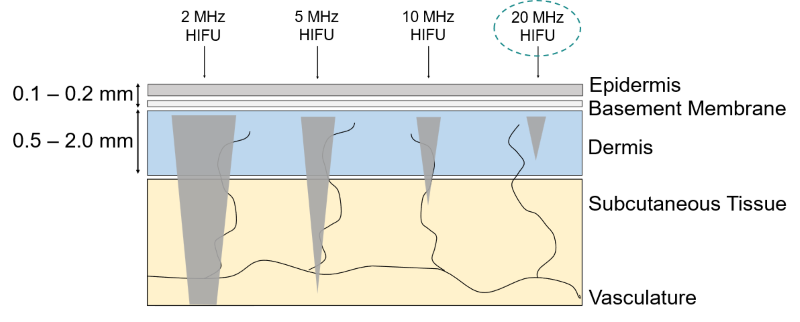
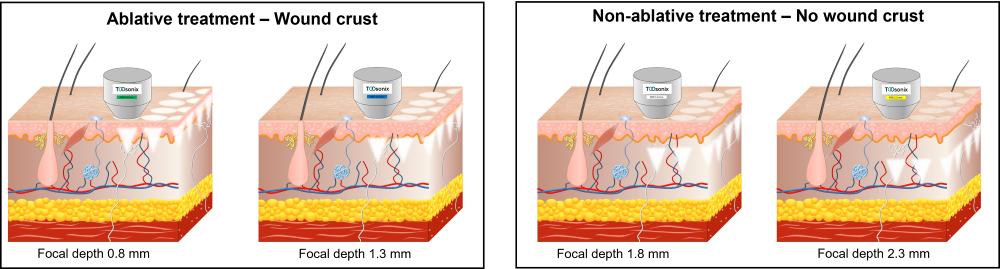
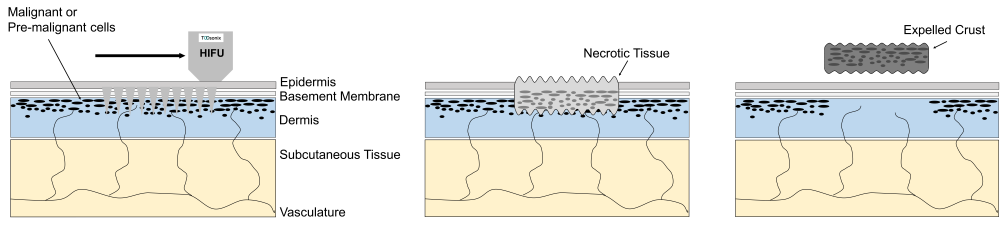
Each handpiece has a calibration curve encoded into its control unit, which calculates and regulates the necessary power settings to obtain a certain selected acoustic energy delivery. The acoustic output energy delivered from the TOOsonix handpiece is linked directly to the selected combination of power and shot duration in the operational menu.
The relation between shot duration, energy level and power level are easily calculated from the below simple formula:
Output energy [J] = Shot duration [s] x Power [W]
The software will use this formula to continuously update the calculated acoustic energy when the shot duration or power level is changed.
Each handpiece is furthermore protected from energy settings that is potentially damaging for the components inside. The handpiece therefore has a pre-defined allowable combinational range between shot duration and power. Please note that the allowable range depends on the type of handpieces and can be different for different types.
Duty Cycle and Shot Interval
The systems from TOOsonix also offer an option for automatic shot repetition at fixed intervals when “Repeated Shot Mode” is selected. The shots are then automatically repeated at fixed intervals that can be adjusted by the user from the operational menu.
The Shot Duration and Shot Interval determines the sonification session Duty Cycle (the percentage of time in which the system actively transmits ultrasound signals).
The TOOsonix systems have been limited to certain minimum intervals in relation to the burst time. Each handpiece has limitations encoded into its control unit to prevent damaging components and potential overheating or degradation. These limitations means that the higher the energy the system operates at, the longer the interval between bursts has to be.
Adjustment of the required Dose Interval is done from the operational menu. The software will use the selection to continuously update the calculated Duty Cycle when the Shot Interval or Shot Duration is changed.