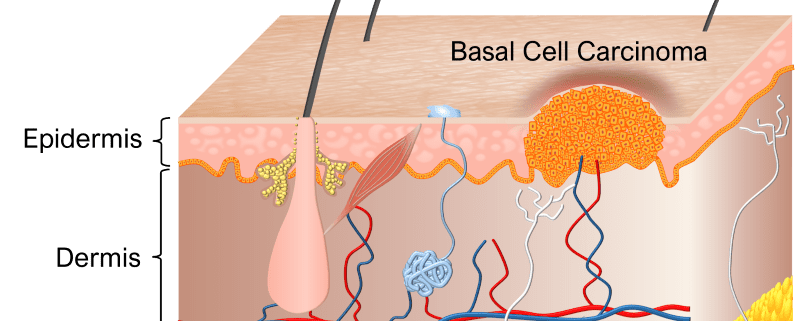
The Danish Medicines Agency and National Committee on Health Research Ethics approved the start of a clinical investigation on single-session removal of basal cell carcinoma (BCC).
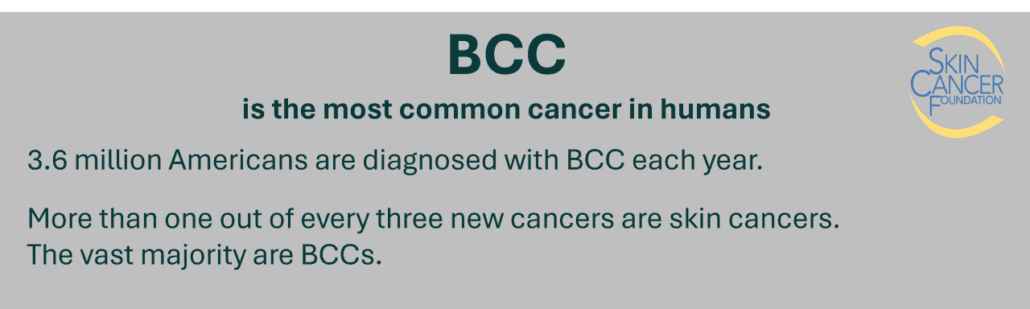
BCC is the most common type of cancer in the world, and treatments are counted in several tens of millions every year. Existing therapies have shortcomings in the form of varying efficacies, high treatment pain, scar formation, burn/freeze damages, pigmentary changes, radiation damages, nerve damages, hair-loss, and pharmaceutical side-effects.
With the new HIFU treatment we believe we can improve this situation.
The study will be conducted as a dual-center GCP investigation following all requirements from the new European Medical Device Regulation. It will be performed at the dermatological departments of Bispebjerg and Roskilde University Hospitals. The teams will recruit participants over the following months, and thereby start treatments in the autumn.
The full follow-up period will be 12 months as in most other cancer studies, but the protocol includes interim reports and updates during the study.
Please wish the two medical teams good luck and keep looking out for the updates on this unique new treatment that can change the lives of millions!